Researchers create new reagents for a fluorinated version of copper-catalyzed azide-alkyne click chemistry
By
Department:
Keywords: , , ,
[+]Enlarge
A two-step, one-pot reaction creates new fluorinated azides for copper-catalyzed fluorinated click chemistry.
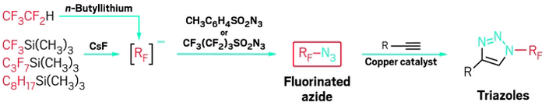
A two-step, one-pot reaction creates new fluorinated azides for copper-catalyzed fluorinated click chemistry.
With the help of new fluorinating reagents, a team led by Zsófia E. Blastik and of the Czech Academy of Sciences has devised a versatile fluorinated version of copper-catalyzed click chemistry. The approach overcomes some previous difficulties with preparing suitable fluorinated reagents for click reactions and opens the door to broader use of click chemistry, which has become invaluable to biochemistry and polymer science researchers.
The new chemistry hinges on the ability to make azidoperfluoroalkanes. Azidotrifluoromethane (CF
3N
3) has been known for some time, but its best reported synthesis starting from CF
3I requires cumbersome handling of toxic and corrosive CF
3NO, N
2H
4, and Cl
2 gases—an approach that has limited CF
3N
3’s applications. Beier’s group alternatively attempted using electrophilic CF
3I with sodium azide (NaN
3) as a nucleophile, but the reaction didn’t work. Instead, the team found that CF
3N
3 and its previously unknown longer chain analogs can be prepared more conveniently from CF
3Si(CH
3)
3 and related nucleophiles and sulfonyl azide electrophiles. In effect, the researchers flipped the polarity of the reaction’s constituents (
Angew. Chem. Int. Ed. 2016, DOI: ).
The reported azidoperfluoroalkanes undergo copper-catalyzed azide-alkyne cycloadditions, also known as click reactions, leading to N-perfluoroalkyl triazoles as underexplored building blocks, Beier says. “In fact, azidoperfluoroalkanes are more reactive in the click reaction with alkynes than nonfluorinated alkyl azides.”
“A reminder emerging out of this paper by Beier and colleagues is that, if you want to build a bond by a polar mechanism, there are always two options,” says of the University of Toronto, who focuses on the . “It is a good idea to reverse the polarity of components if one path is problematic. As a result, they have developed an efficient entry into azidoperfluoroalkanes.”
“This is a very attractive, scalable, one-step method for preparing azidotrifluoroalkanes,” adds of the Shanghai Institute of Organic Chemistry, whose group . The azides display excellent reactivity in click reactions, Shen says, “paving the way for the broad application of azidoperfluoroalkanes in many fields, including but not limited to chemical biology and materials science.”

